Triamcinolone Acetonide Injection IP
MOQ : 1000 Pieces
Triamcinolone Acetonide Injection IP Specification
- Salt Composition
- Triamcinolone Acetonide
- Indication
- Allergic disorders, dermatologic diseases, arthritis, and other conditions responsive to corticosteroid therapy
- Packaging Type
- Vial / Ampoule
- Dosage Form
- Parenteral (Injection)
- Origin of Medicine
- Allopathic
- Pacakaging (Quantity Per Box)
- 5 or 10 ampoules per box
- Drug Type
- General Medicines
- Ingredients
- Triamcinolone Acetonide
- Physical Form
- Tablets
- Function
- Corticosteroid injection for intra-articular, intramuscular, and soft tissue administration
- Recommended For
- Anti-inflammatory and immunosuppressive therapy
- Dosage
- As directed by physician
- Dosage Guidelines
- As per suggestion
- Suitable For
- Adults and children as advised
- Quantity
- 1 ml, 2 ml (varies by pack)
- Storage Instructions
- Dry Place
- Strength
- 10 mg/ml or 40 mg/ml (as per pack)
- Shelf Life
- 24 months from the date of manufacture
- Batch Number
- Available on vial/ampoule
- Prescription Required
- Yes
- Route of Administration
- Intramuscular, Intra-articular, Intralesional
- Side Effects
- Injection site reactions, adrenal suppression, potential systemic effects
- Color
- Clear to slightly milky suspension
- Marketed By
- As per pack or manufacturer
Triamcinolone Acetonide Injection IP Trade Information
- Minimum Order Quantity
- 1000 Pieces
- Payment Terms
- Cash in Advance (CID)
- Supply Ability
- 1000 Pieces Per Day
- Delivery Time
- 5 Days
- Main Domestic Market
- All India
About Triamcinolone Acetonide Injection IP
Experience the resplendent efficacy of Triamcinolone Acetonide Injection IP-an advanced anti-inflammatory and immunosuppressive corticosteroid, available in strengths of 10 mg/ml or 40 mg/ml. This sensational injection, presented as a clear to slightly milky suspension, is ideal for intra-articular, intramuscular, or intralesional administration. Get it now for a limited time and enjoy first-rate relief from allergic disorders, dermatologic diseases, and arthritis. Prescription is required. Store in a dry place, and always follow the physician's dosage. Packaging varies, available in vials or ampoules.
Applications and Areas of Use
Triamcinolone Acetonide Injection IP is employed for its potent anti-inflammatory and immunosuppressive actions in allergic disorders, dermatologic diseases, and arthritis. The application media is a clear to slightly milky suspension, suitable for direct parenteral administration. It is applied intramuscularly, intra-articularly, or intralesionally in both adult and pediatric patients as advised by a healthcare provider. Plant application refers to sterile pharmaceutical manufacturing processes ensuring quality and consistency.
Export Markets, Certifications, and Payment Terms
Triamcinolone Acetonide Injection IP commands a high rate in both domestic markets and international drop-off destinations, primarily throughout India and major export markets. Batch certifications and compliance with allopathic standards guarantee safety and effectiveness. Flexible payment terms are available for dealers, distributors, exporters, suppliers, traders, and wholesalers, ensuring a seamless purchasing process.
Applications and Areas of Use
Triamcinolone Acetonide Injection IP is employed for its potent anti-inflammatory and immunosuppressive actions in allergic disorders, dermatologic diseases, and arthritis. The application media is a clear to slightly milky suspension, suitable for direct parenteral administration. It is applied intramuscularly, intra-articularly, or intralesionally in both adult and pediatric patients as advised by a healthcare provider. Plant application refers to sterile pharmaceutical manufacturing processes ensuring quality and consistency.
Export Markets, Certifications, and Payment Terms
Triamcinolone Acetonide Injection IP commands a high rate in both domestic markets and international drop-off destinations, primarily throughout India and major export markets. Batch certifications and compliance with allopathic standards guarantee safety and effectiveness. Flexible payment terms are available for dealers, distributors, exporters, suppliers, traders, and wholesalers, ensuring a seamless purchasing process.
FAQ's of Triamcinolone Acetonide Injection IP:
Q: How should Triamcinolone Acetonide Injection IP be administered?
A: This injection should be administered intramuscularly, intra-articularly, or intralesionally by a healthcare professional, as per the physician's guidelines.Q: What conditions benefit from Triamcinolone Acetonide Injection IP?
A: It is recommended for allergic disorders, dermatologic diseases, arthritis, and other conditions responsive to corticosteroid therapy.Q: When is a prescription required for this medication?
A: A prescription is mandatory; always consult a licensed healthcare provider before using Triamcinolone Acetonide Injection IP.Q: Where can I find information on the batch number and shelf life?
A: Batch number and manufacturing details are printed on the vial or ampoule, with a shelf life of 24 months from the date of manufacture.Q: What are the storage instructions for Triamcinolone Acetonide Injection IP?
A: Store the product in a dry place, away from direct light and moisture, as per packaging instructions.Q: What is the process for bulk purchasing for dealers or wholesalers in India?
A: Dealers, distributors, and wholesalers can contact the supplier directly, review certifications, agree on payment terms, and arrange drop-off or shipping as required.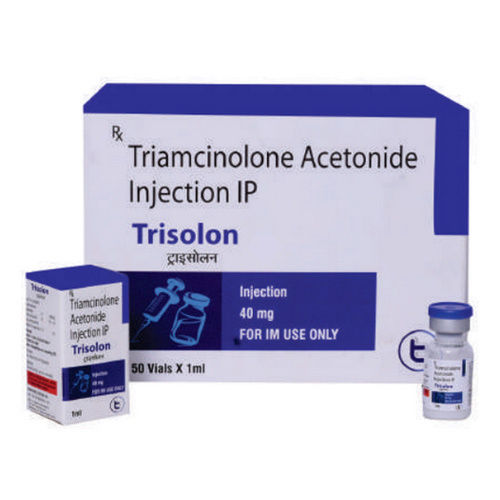
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Pharmaceutical Injection Category
Frusemide Injection IP
Minimum Order Quantity : 100 Units
Drug Type : Injection
Storage Instructions : Dry Place
Physical Form : Liquid
Dosage : As per suggestion
Recommended For : Human Being
Adrenaline Injection IP
Minimum Order Quantity : 10000 Units
Drug Type : Injection
Storage Instructions : Dry Place
Physical Form : Liquid
Dosage : As per suggestion
Recommended For : Human Being
Ondansetron Hydrochloride Injection IP
Minimum Order Quantity : 10000 Units
Drug Type : Injection
Storage Instructions : Dry Place
Physical Form : Liquid
Dosage : As per suggestion
Recommended For : Human Being
Tranexamic Acid Injection IP
Minimum Order Quantity : 5000 Pieces
Drug Type : Injection
Storage Instructions : Dry Place
Physical Form : Liquid


 Send Inquiry
Send Inquiry

 Send Inquiry
Send Inquiry Send SMS
Send SMS English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese