Diclofenac Sodium Injection IP
Diclofenac Sodium Injection IP Specification
- Pacakaging (Quantity Per Box)
- 5 x 3 ml ampoules
- Indication
- Musculoskeletal disorders, postoperative pain, rheumatoid arthritis
- Salt Composition
- Diclofenac Sodium 75 mg
- Packaging Type
- Ampoule
- Origin of Medicine
- Indian
- Dosage Form
- Injection
- Drug Type
- Injection
- Ingredients
- Diclofenac Sodium IP
- Physical Form
- Liquid
- Function
- Analgesic and Anti-inflammatory
- Recommended For
- Relief of pain and inflammation
- Dosage
- 75 mg/3 ml
- Dosage Guidelines
- As per suggestion
- Suitable For
- Adults
- Quantity
- 3 ml ampoule
- Storage Instructions
- Dry Place
- Administration Route
- Intramuscular injection
- Prescription/Non Prescription
- Prescription
- pH Range
- 7.0 to 8.0
- Shelf Life
- 2 years from manufacturing date
- Appearance
- Clear, colorless to faint yellow solution
Diclofenac Sodium Injection IP Trade Information
- Minimum Order Quantity
- 10000 Vials
- Payment Terms
- Cash in Advance (CID)
- Supply Ability
- 1000 Vials Per Day
- Delivery Time
- 5 Days
- Main Domestic Market
- All India
About Diclofenac Sodium Injection IP
Use Type, Area & Usage Type of Diclofenac Sodium Injection IP
Diclofenac Sodium Injection IP is intended for professional intramuscular administration, ensuring rapid and effective relief for adults suffering from pain and inflammation. Suitable for use in hospitals, clinics, and outpatient settings, this prescription medication targets a broad area of application, including orthopedic, postoperative, and rheumatologic pain management. Its usage type is strictly as directed by a registered medical practitioner, guaranteeing both safety and superior therapeutic results.
Drop-off, Charge & Export Packaging for Diclofenac Injection
Diclofenac Sodium Injection IP is meticulously packaged in 3 ml ampoules, with each carton containing 5 x 3 ml ampoules, ensuring safe drop-off during transit. We offer sample availability upon request and maintain competitive charges for bulk and wholesale orders. Products are shipped promptly to all key export markets, including Asia, Africa, the Middle East, and more, upholding optimum quality standards throughout the logistics process for our international clientele.
FAQ's of Diclofenac Sodium Injection IP:
Q: How should Diclofenac Sodium Injection IP be administered?
A: Diclofenac Sodium Injection IP must be administered intramuscularly by a qualified healthcare provider, following the recommended dosage and instructions provided by a physician.Q: What are the primary uses and benefits of this injection?
A: This injection is chiefly used for the relief of pain and inflammation related to musculoskeletal disorders, postoperative pain, and rheumatoid arthritis, providing quick and noteworthy symptomatic improvement.Q: When is it appropriate to use Diclofenac Sodium Injection IP?
A: It is appropriate for use when rapid and potent analgesic or anti-inflammatory intervention is required, as advised by a health professional.Q: Where should Diclofenac Sodium Injection IP be stored?
A: Store the ampoules in a dry place at controlled room temperature, away from direct sunlight, to maintain product stability over its two-year shelf life.Q: What is the process for obtaining Diclofenac Sodium Injection IP?
A: As a prescription medication, Diclofenac Sodium Injection IP is available through registered dealers, distributors, exporters, suppliers, and traders. A valid prescription is required for purchase and use.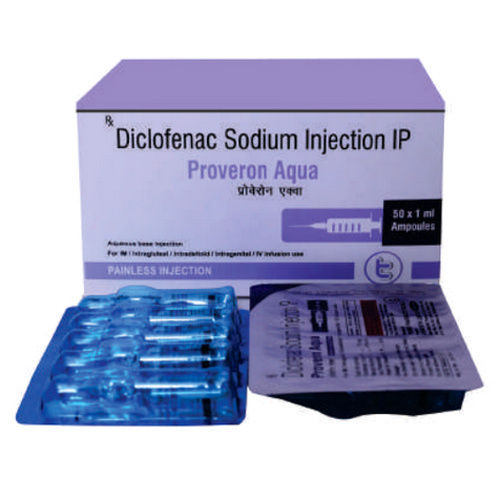

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Pharmaceutical Injection Category
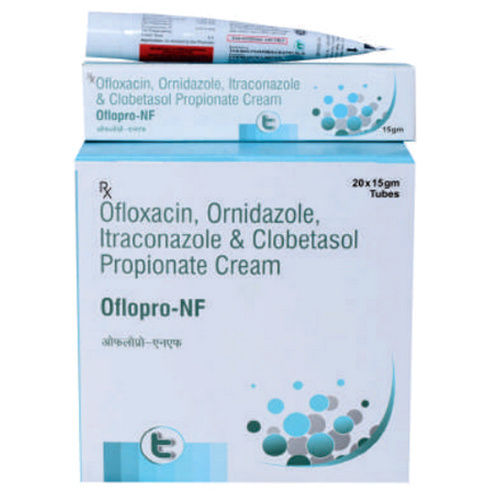
Ofloxacin Ornidazole Itraconazole And Clobetasol Propionate Cream
Minimum Order Quantity : 1000 Pieces
Drug Type : General Medicines
Physical Form : Other, Cream
Storage Instructions : Dry Place
Recommended For : Bacterial and fungal skin infections
Dosage : As prescribed by physician
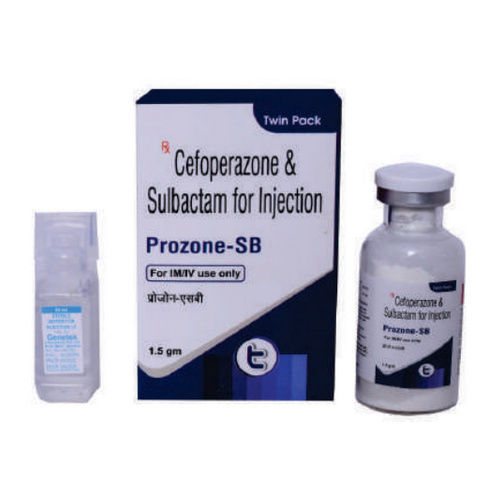
Cefoperazone And Sulbactam For Injection
Minimum Order Quantity : 1000 Vials
Drug Type : General Medicines
Physical Form : Other, Powder for Injection
Storage Instructions : Dry Place
Recommended For : Bacterial Infections
Dosage : 1.5 gm
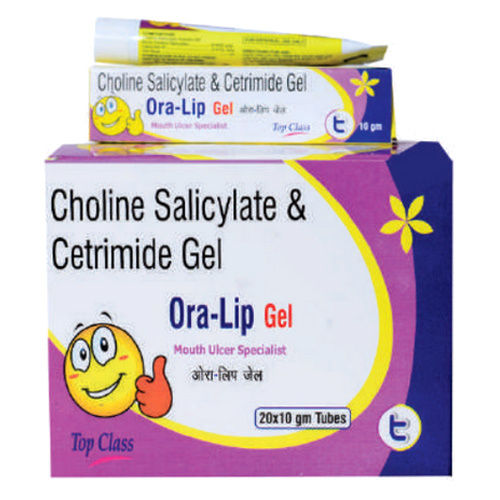
Choline Salicylate And Cetrimie Gel
Minimum Order Quantity : 1000
Drug Type : Injection
Physical Form : Other, Gel
Storage Instructions : Dry Place
Recommended For : Oral and topical use
Dosage : As directed by physician
Ceftriaxone And Sulbactam For Injection IP
Minimum Order Quantity : 100 Units
Drug Type : Injection
Physical Form : Liquid
Storage Instructions : Dry Place
Recommended For : Human Being
Dosage : As per suggestion


 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese